Ocean Acidification Due to Elevated Levels of Carbon Dioxide Prohibits Reef Growth

Artificially acidified seawater is released over the reefs, with a red dye to monitor distribution (Photo: Aaron Takeo Ninokawa)
In the wake of the mass coral bleaching events over the past few years, a new study has found that ocean acidification, caused by elevated levels of atmospheric carbon dioxide dissolving into the ocean, may have a detrimental impact on the ability of coral reefs to regenerate in the future.
Mixed with water, CO2 forms carbonic acid, which is capable of dissolving the calcium carbonate ‘skeletons’ that are formed by reef-building corals during their metabolic cycle. Concentrations of the acid in the world’s oceans are not high enough to reduce the extent of the reef by itself, but they do slow the rate of ‘calcification’ – the process by which the skeletons are formed – and therefore inhibit the growth of new reef structures. According to the report, published this week in Nature, this ‘[reduction in] calcification, coupled with increased bioerosion and dissolution, may drive reefs into a state of net loss this century.’
The acidity or alkalinity (technically ‘basicity’) is measured on the pH scale. Solutions with a pH level of 7 (such as pure water) are non-reactive, anything with a pH level under 7 is classed as ‘acidic’, and anything over 7 is classed as ‘alkaline’ or ‘basic’. The current pH level of the world’s oceans averages at 8.1, a reduction from the pH level of 8.2 associated with the pre-industrial era.
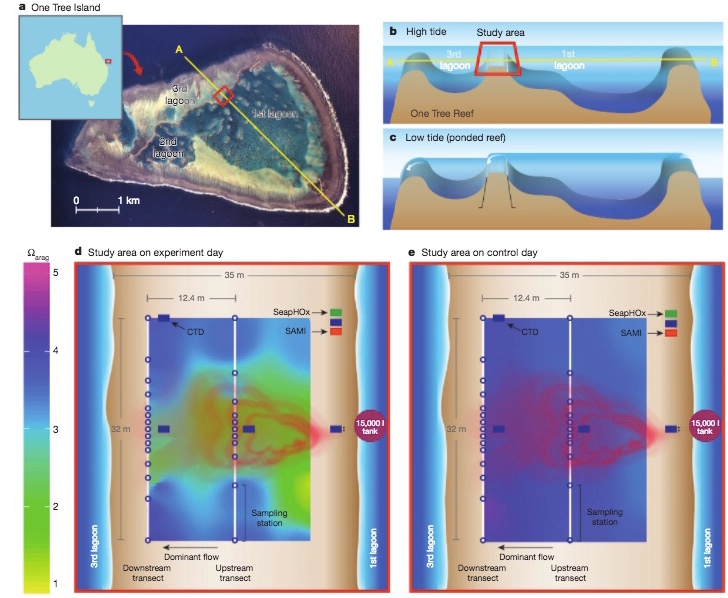
To perform the experiment, the team of researchers constructed a tank, filled with seawater, which was saturated with carbon dioxide, and released it over 400 square meters of reef near One Tree Island, in the southern part of the Great Barrier Reef. This reduced the pH level of the surrounding seawater to just under 8.0, which is the predicted pH level of the ocean by 2060, should atmospheric CO2 continue to increase at its current rate.
The researchers, led by Dr. Rebecca Albright, assistant curator at the California Academy of Science and Ken Caldeira of the Carnegie Institution of Science, found that the rate of calcification decreased by as much as 30 percent, a worrying figure based on only a slight reduction in pH levels. Dr. Albright said that this was much greater than the 15 percent found in previous studies, but also noted that those studies had been conducted on individual corals in a laboratory, and not on an open reef.
‘Coral reefs are so much more than just corals,’ said Dr. Albright. ‘We’re really looking at the cumulative response of the entire system, not just coral.’

The Great Barrier Reef has been hit by back-to-back bleaching events
The new research follows a 2016 experiment by the same team in which they increased the alkalinity of the sea water surrounding a coral reef to reflect the pH level of 8.2 from the pre-industrial era. There, they found that the rate of calcification increased with the change in the sea water’s chemical composition.
Recent coral bleaching events have demonstrated that reef recovery is already jeopardized, as a rapid succession of El Nino events has demonstrated. Although the acidification process does not cause reef death in the same way as bleaching, Dr. Albright notes that ‘temperature and ocean acidification are acting in tandem at different scales and on different processes, but they’re both eroding away the resilience of these systems,’
The impact on reefs in years to come remains uncertain, but there are a growing number of indications that the recovery of damaged reefs will be compromised well into the future. ‘Coral reefs offer economic opportunities to their surrounding communities from fishing and tourism,’ said Dr. Caldeira. ‘But for me, the reef is a beautiful and diverse outpouring of life that we are harming with our carbon dioxide emissions. For the denizens of the reef, there’s not a moment to lose in building an energy system that doesn’t dump its waste into the sky or sea.’
Adapted from Dive Magazine www.divemagazine.co.uk



Leave a Reply
Your email is safe with us.